First prototype of a fully bioresorbable all-solid-state Na-ion microbattery

February 28, 2025

The SIMBA project presents a first prototype of a fully bioresorbable all-solid-state Na-ion microbattery.
Thierry DJENIZIAN (EMSE)
The SIMBA project aims to design a flexible, implantable, and fully bioresorbable sodium-ion microbattery.
The development of biocompatible batteries is crucial for powering temporary implantable medical devices and, more broadly, bioelectronic systems for the Internet of Things dedicated to healthcare. However, currently used batteries, such as lithium-ion systems, are toxic and cannot be safely broken down naturally by the human body. Their use therefore necessitates removal through a second surgical procedure at the end of their intended lifespan.
In this context, recent efforts have focused on designing bioresorbable batteries based on alternative chemistries. Unfortunately, these have so far shown limited performance, are non-rechargeable, bulky, and difficult to integrate. The sodium-ion technology developed within the SIMBA project offers a promising alternative, as the components of these rechargeable batteries can be biocompatible.
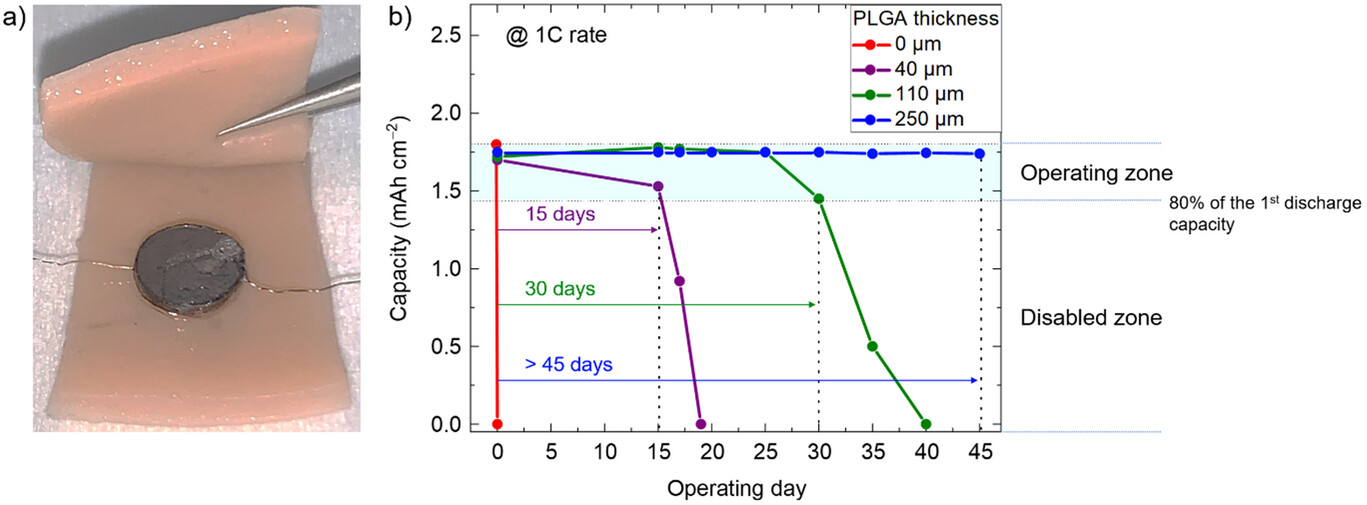
During the first year of the project, an innovative manufacturing process was implemented to develop a fully bioresorbable prototype with excellent electrochemical performance (discharge capacity of 5.1 mAh cm⁻²). Initial degradation tests under in vitro and in vivo conditions showed no signs of toxicity from the breakdown products. Made entirely of biocompatible materials, this fully solid-state rechargeable battery can thus be safely decomposed by biological fluids.
Moreover, the lifespan of this new energy storage system after implantation can be finely controlled by adjusting the thickness of the encapsulation layer. This property enables the design of “on-demand” bio-eliminable batteries, capable of operating from a few days to several weeks.
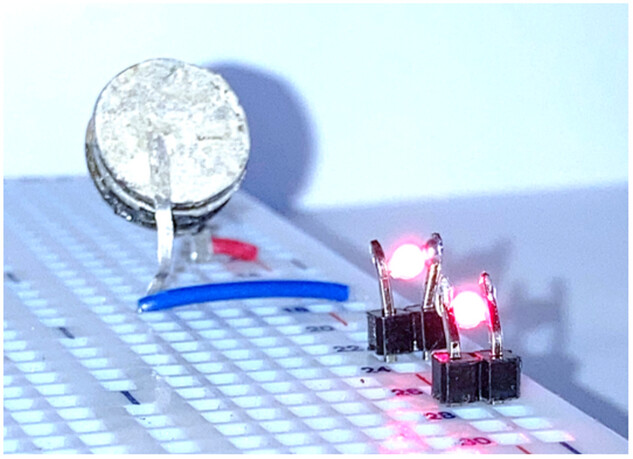
Preliminary cytotoxicity tests tend to confirm the safety of the device. Additionally, wireless charging of the battery through the skin has been demonstrated using an inductive charger to prove the viability of the approach.
These results are published in Advanced Functional Materials (https://doi.org/10.1002/adfm.202417353).
Autres Highlights